
Welcome to ARS—your gateway to the exciting world of Rhenium wonders. We’re not just your typical supplier; we’re your partners in success. At ARS, we’re passionate about creating top-notch Rhenium products.
We take pride in our commitment to delivering quality Rhenium solutions. Our journey is all about striving for perfection to provide you with reliable products that cater to your specific needs. As an excellent member of the industry, we bring together expertise and innovation to offer customized Rhenium alloys. These alloys play a crucial role in fueling advancements across different sectors.
Join us in discovering the brilliance of Rhenium. From aerospace innovations to state-of-the-art electronics, our products empower industries to achieve new milestones. Join us on a limitless journey of excellence, as we shape a future powered by the remarkable abilities of Rhenium.
What is Rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth’s crust.
Rhenium is a silvery-white metal with one of the highest melting points of all elements, exceeded by only tungsten. It also has one of the highest boiling points of all elements, and the highest among stable elements. It is also one of the densest, exceeded only by platinum, iridium and osmium. Rhenium has a hexagonal close-packed crystal structure, with lattice parameters a = 276.1 pm and c = 445.6 pm.
Its usual commercial form is a powder, but this element can be consolidated by pressing and sintering in a vacuum or hydrogen atmosphere. This procedure yields a compact solid having a density above 90% of the density of the metal. When annealed this metal is very ductile and can be bent, coiled, or rolled. Rhenium-molybdenum alloys are superconductive at 10 K; tungsten-rhenium alloys are also superconductive around 4–8 K, depending on the alloy. Rhenium metal superconducts at 1.697±0.006 K.
In bulk form and at room temperature and atmospheric pressure, the element resists alkalis, sulfuric acid, hydrochloric acid, nitric acid, and aqua regia. It will however, react with nitric acid upon heating.
Explore Our Rhenium Products
Explore the Diversity of Rhenium Solutions
Discover the many faces of Rhenium excellence at ARS. We take pride in offering a variety of Rhenium products, such as Rhenium powder, Rhenium plate, Rhenium bar, Rhenium alloys, Rhenium pellet, Rhenium Foil and so on, that fit the unique needs of different industries. Our extensive catalog also showcases the versatility of Rhenium, ensuring that whatever industry you are in, we have the perfect Rhenium solution for you.
Tailored to You: Customized Rhenium Services
At ARS, we know one size doesn’t fit all. That’s why we provide personalized Rhenium services that meet expectations. Our expert team collaborates closely with clients to understand your specific needs, crafting customized Rhenium solutions that seamlessly integrate into your processes. Experience the power of tailored excellence with ARS, where your success is our priority.
Why Choose ARS-Our Advantages
Physical Properties of Rhenium
| Density | lb/in3 | 0.77 |
| gm/cm3 | 21.02 | |
| Melting Point | °F | 5767 |
| °C | 3186 | |
| Boiling Point | °F | 10,170 |
| °C | 5630 | |
| Thermal Conductivity | Cal/cm2/cm°C/sec | 0.39 |
| Specific Heat | Cal/gm/°C | 0.033 |
| Coefficient of Linear Thermal Expansion | micro-in/°F x 10-6 | 11.77 |
| micro-in/°C x 10-6 | 6.5 | |
| Electrical Resistivity | micro-ohm-cm | 13.5 |
Mechanical Properties of Rhenium
| Rhenium Mechanical Properties | ||
|---|---|---|
| Tensile Strength | KSI (Mpa)-RT | 200 (1380) |
| KSI (Mpa)-500°C | 135 (930) | |
| KSI (Mpa)-1000°C | 70 (480) | |
| Elongation | % in 1.0″. | 2 |
| Hardness | DPH | — |
| Modulus of Elasticity | KSI | 67150 |
| Gpa | 463 | |
Make Rhenium and Its Products
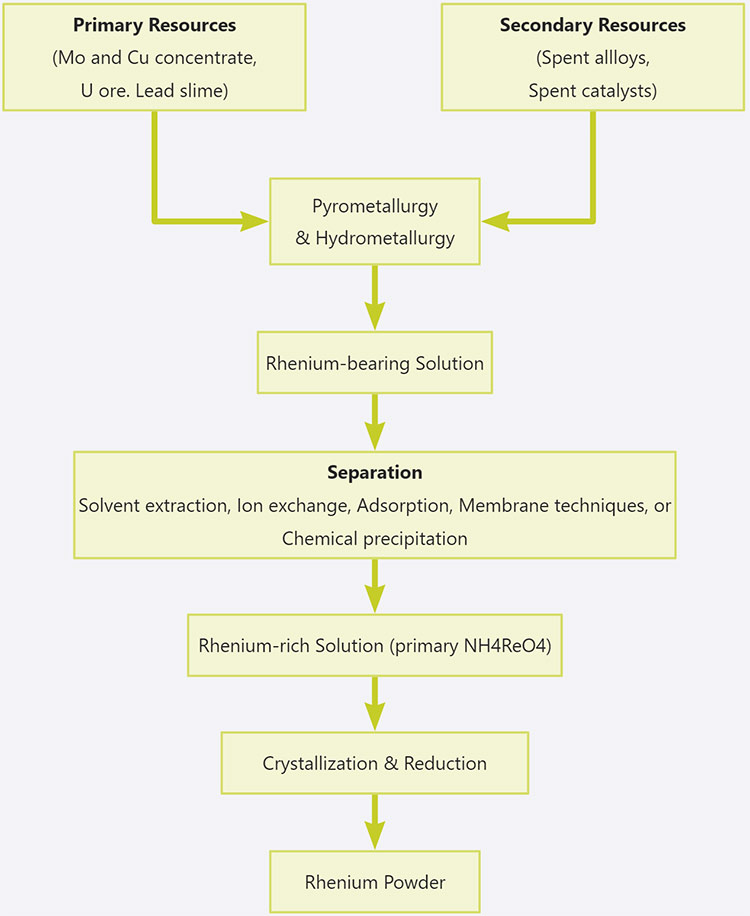
Rhenium is mainly produced from the by-products of molybdenum and copper smelting processes. When extracting rhenium, first prepare a rhenium-containing solution from the rhenium-containing raw material. The solution is separated and purified to extract the pure rhenium compound. Then, the hydrogen reduction method, aqueous solution electrolysis method, and halide thermal dissociation method are used to prepare rhenium powder, and then the rhenium powder is processed into materials by powder metallurgy and other methods.

High-temperature Sintering Method
This is one of the commonly used industrial methods, including the general process of powder metallurgy, so it is also called powder metallurgy method. First, rhenium powder is put into a steel mold, pressed into rectangular strips at a pressure of 6MPa, and then pre-sintered in vacuum or hydrogen at 1473K for 1 hour to make it have certain strength, and conductivity and to remove some volatile impurities. Finally, the pre-sintered strips are loaded into a furnace at 2973-3123K for high-temperature sintering to achieve high density.
Smelting Method
This method uses sintered bars as raw materials and uses arc melting, electron beam melting and zone melting methods to purify and refine crude rhenium. The rhenium ingot obtained by electron beam melting is a columnar crystal with a purity of 99.99%; the product obtained by zone melting is a rhenium single crystal with spectral purity.
Flotation Method
This is a process that uses differences in the physical and chemical properties of mineral surfaces to separate rhenium minerals. It can separate rhenium minerals from other minerals to obtain pure rhenium materials.
Oxidation Roasting Method and Sulfuric Acid Reduction Method
Both methods are used to extract rhenium from the ore. The oxidation roasting method is to oxidize rhenium ore at high temperature to convert it into rhenium oxide, and then reduce it to metallic rhenium through a reduction reaction. The sulfuric acid reduction method is to leach rhenium ore to obtain a rhenium-containing sulfate solution, which is then reduced to metallic rhenium through a reduction reaction.
Electrolytic Method and Chemical Reduction Method
Both methods can be used for rhenium refining. Electrolysis is to deposit rhenium on the cathode by electrolysis of the rhenium-containing electrolyte, and then dry and sinter it to obtain pure rhenium. The chemical reduction method, on the other hand, is to react the rhenium-containing chemicals with a reducing agent to reduce rhenium to rhenium metal, and then obtain pure rhenium through drying, sintering and other treatments.
Quality Assurance of Rhenium

Chemical Analysis:
Conduct chemical analysis to verify the purity of Rhenium. Use techniques like X-ray fluorescence (XRF) or inductively coupled plasma mass spectrometry (ICP-MS) to determine elemental composition.
Microstructural Examination:
Perform microscopy to examine the microstructure of Rhenium. Check for grain size, uniformity, and potential impurities that may affect performance.
Mechanical Testing:
Conduct mechanical tests, such as tensile and compression testing, to assess the strength and deformation properties of Rhenium. Evaluate hardness and other mechanical characteristics.
Density Measurement:
Verify the density of Rhenium using methods like Archimedes’ principle or gas pycnometry. Ensure that the measured density aligns with the expected value for pure Rhenium. Get A Fast Quote
Get A Fast Quote
Radiographic Inspection:
Utilize radiographic testing to identify internal defects or inconsistencies in the structure of Rhenium. This method is particularly useful for detecting hidden flaws that may impact performance.
Non-Destructive Testing (NDT):
Employ NDT methods like ultrasonic testing or eddy current testing to assess the integrity of Rhenium without causing damage.
Thermal Analysis:
Perform thermal analysis, including differential scanning calorimetry (DSC) or thermogravimetric analysis (TGA), to assess thermal stability and behavior.
Spectroscopic Analysis:
Use spectroscopic techniques, such as X-ray photoelectron spectroscopy (XPS) or Fourier-transform infrared spectroscopy (FTIR), to analyze surface chemistry and identify any surface contaminants.
Rhenium Applications Across Industries

High-temperature Alloys
Rhenium is the most widely used in high-temperature alloys. Rhenium can form high-temperature alloys with tungsten, iron, nickel, cobalt and other metal elements to improve the strength, hardness, corrosion resistance and oxidation resistance of the alloy. Especially in the aerospace field, rhenium’s high-temperature strength and corrosion resistance are fully utilized. For example, rhenium alloys can be used to manufacture key components such as turbine blades and combustion chambers of aerospace engines, improving engine performance and reliability.

Electronic and Optical Materials
Rhenium’s high electrical resistance and good thermal stability make it ideal for electronic and optical materials. Rhenium can be used to manufacture electronic devices at high temperatures, such as electrical connectors, resistors, capacitors, etc. In the field of optics, rhenium has high refractive index and low dispersion properties and can be used to manufacture high-quality optical lenses and optical fibers.

Catalyst
Rhenium has excellent catalytic properties and can be used in the field of catalysts. For example, rhenate can be used as an active ingredient in petroleum cracking catalysts to effectively improve the yield and quality of petroleum. In addition, rhenium can also form catalysts with other metals. For example, platinum-rhenium catalysts can be used in reactions such as automobile exhaust purification and chemical synthesis.

Medical and Biological Fields
Rhenium also has a wide range of applications in the medical and biological fields. For example, rhenium can be used together with radioactive elements to form radiopharmaceuticals for tumor treatment and diagnosis. In addition, rhenium can also be used for labeling and tracing in organisms to study material metabolism and physiological processes in organisms.
Health Effects of Rhenium
It is very little known about rhenium toxicity.
Potential health effects: May cause eye irritation and skin irritation. The liquid state may cause burns to the skin and eyes.
Ingestion: May cause irritation of the digestive tract.
Inhalation: May cause respiratory tract irritation.
The toxicological properties of this substance have not been thoroughly investigated. Vapors may cause dizziness or suffocation.












